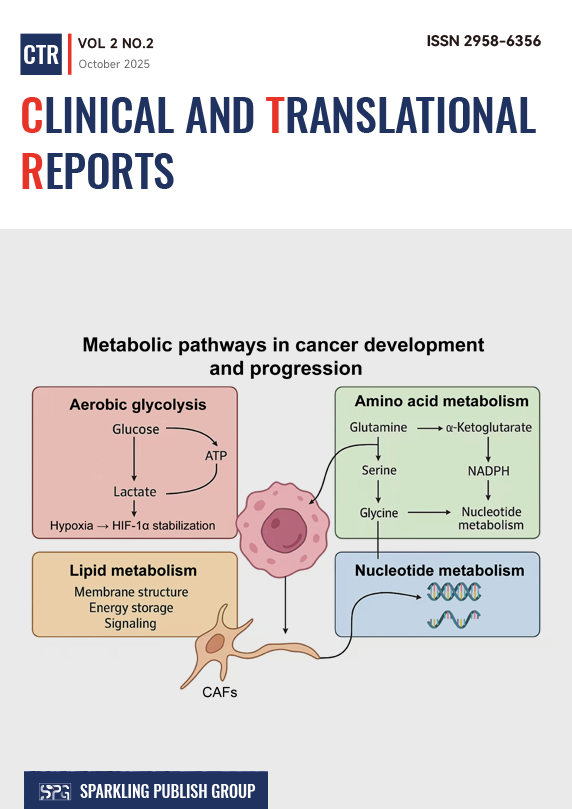
Metabolic reprogramming has emerged as a defining hallmark of cancer, reflecting the capacity of malignant cells to restructure bioenergetic and biosynthetic pathways in response to genetic alterations and microenvironmental pressures. Tumor cells exploit metabolic plasticity to sustain proliferation, maintain redox homeostasis, and adapt to nutrient fluctuations or hypoxia. Glucose metabolism is frequently diverted toward aerobic glycolysis, yielding both ATP and intermediates essential for macromolecule synthesis. In parallel, glutamine catabolism replenishes tricarboxylic acid cycle intermediates and supports nucleotide production, whereas lipid and amino acid metabolism undergo extensive remodeling to provide structural components and signaling molecules that promote tumor growth. Such multifaceted alterations integrate with oncogenic signaling networks, creating a finely tuned metabolic landscape that underlies malignancy. Metabolic reprogramming extends beyond intrinsic tumor processes to reshape the tumor microenvironment (TME). Excessive consumption of glucose and glutamine by cancer cells restricts nutrient access for effector immune populations, fostering immune suppression. Accumulated metabolites such as lactate, kynurenine, and adenosine acidify the extracellular milieu, polarize macrophages toward tumor-supportive phenotypes, and dampen cytotoxic T-cell activity. Cancer-associated fibroblasts further participate through metabolic symbiosis, providing energy-rich substrates that sustain tumor oxidative metabolism and angiogenesis. Hypoxia-driven stabilization of hypoxia-inducible factors reinforces glycolytic flux and vascular remodeling, perpetuating a self-sustaining cycle of metabolic and microenvironmental adaptation. Understanding these metabolic interactions can provide a deeper understanding of cancer pathophysiology and may also reveal exploitable vulnerabilities. Therapeutic strategies targeting metabolic enzymes or normalizing nutrient fluxes represent promising avenues for disrupting tumor–stroma cooperation and enhancing treatment efficacy.
1. Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021;10(5):1056.
2. Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Molecular cancer. 2021;20(1):28.
3. Zhang H, Li S, Wang D, Liu S, Xiao T, Gu W, et al. Metabolic reprogramming and immune evasion: the interplay in the tumor microenvironment. Biomarker research. 2024;12(1):96.
4. Nicolini A, Ferrari P. Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy. Frontiers in Immunology. 2024;15:1353787.
5. Cassim S, Pouyssegur J. Tumor microenvironment: a metabolic player that shapes the immune response. International journal of molecular sciences. 2019;21(1):157.
6. Piwocka O, Piotrowski I, Suchorska WM, Kulcenty K. Dynamic interactions in the tumor niche: how the cross-talk between CAFs and the tumor microenvironment impacts resistance to therapy. Frontiers in Molecular Biosciences. 2024;11:1343523.
7. Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Archives of pharmacal research. 2019;42(10):833-47.
8. Bott AJ, Maimouni S, Zong W-X. The pleiotropic effects of glutamine metabolism in cancer. Cancers. 2019;11(6):770.
9. Li H, Feng Z, He M-L. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics. 2020;10(16):7053.
10. Mullen NJ, Singh PK. Nucleotide metabolism: a pan-cancer metabolic dependency. Nature Reviews Cancer. 2023;23(5):275-94.
11. Li X, Zhang HS. Amino acid metabolism, redox balance and epigenetic regulation in cancer. The FEBS Journal. 2024;291(3):412-29.
12. Multhoff G, Vaupel P. Lactate-avid regulatory T cells: metabolic plasticity controls immunosuppression in tumour microenvironment. Signal Transduction and Targeted Therapy. 2021;6(1):171.
13. Zhu L, Zhu X, Wu Y. Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules. 2022;12(4):580.
14. Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Experimental & molecular medicine. 2020;52(9):1496-516.
15. Erazo‐Oliveras A, Muñoz‐Vega M, Salinas ML, Wang X, Chapkin RS. Dysregulation of cellular membrane homeostasis as a crucial modulator of cancer risk. The FEBS journal. 2024;291(7):1299-352.
16. Zhao H, Li Y. Cancer metabolism and intervention therapy. Molecular Biomedicine. 2021;2(1):5.
17. Kierans S, Taylor C. Regulation of glycolysis by the hypoxia‐inducible factor (HIF): implications for cellular physiology. The Journal of physiology. 2021;599(1):23-37.
18. Su X, Yang Y, Yang Q, Pang B, Sun S, Wang Y, et al. NOX4-derived ROS-induced overexpression of FOXM1 regulates aerobic glycolysis in glioblastoma. BMC cancer. 2021;21(1):1181.
19. Lu Y, Ma H, Xiong X, Du Y, Liu L, Wang J, et al. Deletion of ENO1 sensitizes pancreatic cancer cells to gemcitabine via MYC/RRM1-mediated glycolysis. Scientific Reports. 2025;15(1):9941.
20. Deng H, Chen Y, Li P, Hang Q, Zhang P, Jin Y, et al. PI3K/AKT/mTOR pathway, hypoxia, and glucose metabolism: potential targets to overcome radioresistance in small cell lung cancer. Cancer Pathogenesis and Therapy. 2023;1(01):56-66.
21. Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, et al. Lactate modulates CD4+ T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38(19):3681-95.
22. Rawat D, Chonker SK, Naik RA, Mehrotra A, Trigun SK, Koiri RK. Lactate as a signaling molecule: Journey from dead end product of glycolysis to tumor survival. Frontiers in Bioscience-Landmark. 2019;24(2):366-81.
23. Ni R, Li Z, Li L, Peng D, Ming Y, Li L, et al. Rethinking glutamine metabolism and the regulation of glutamine addiction by oncogenes in cancer. Frontiers in Oncology. 2023;13:1143798.
24. Zhong X, He Z, Yin L, Fan Y, Tong Y, Kang Y, et al. Glutamine metabolism in tumor metastasis: Genes, mechanisms and the therapeutic targets. Heliyon. 2023;9(10).
25. Alhallaq AS, Sultan NS. Fueling Prostate Cancer: The Central Role of Glutamine/Glutamate Metabolic Reprogramming. Asian Pacific Journal of Cancer Prevention. 2025;26(9):3157-74.
26. Murtas G, Marcone GL, Sacchi S, Pollegioni L. L-serine synthesis via the phosphorylated pathway in humans. Cellular and Molecular Life Sciences. 2020;77(24):5131-48.
27. Luo W, Zou Z, Nie Y, Luo J, Ming Z, Hu X, et al. ASS1 inhibits triple-negative breast cancer by regulating PHGDH stability and de novo serine synthesis. Cell Death & Disease. 2024;15(5):319.
28. Montrose DC, Saha S, Foronda M, McNally EM, Chen J, Zhou XK, et al. Exogenous and endogenous sources of serine contribute to colon cancer metabolism, growth, and resistance to 5-fluorouracil. Cancer research. 2021;81(9):2275-88.
29. Sánchez-Castillo A, Heylen E, Hounjet J, Savelkouls KG, Lieuwes NG, Biemans R, et al. Targeting serine/glycine metabolism improves radiotherapy response in non-small cell lung cancer. British Journal of Cancer. 2024;130(4):568-84.
30. Barnabas GD, Lee JS, Shami T, Harel M, Beck L, Selitrennik M, et al. Serine biosynthesis is a metabolic vulnerability in IDH2-driven breast cancer progression. Cancer research. 2021;81(6):1443-56.
31. Jasani N, Xu X, Posorske B, Kim Y, Wang K, Vera O, et al. PHGDH Induction by MAPK Is Essential for Melanoma Formation and Creates an Actionable Metabolic Vulnerability. Cancer Research. 2025;85(2):314-28.
32. Yang C, Zhang J, Liao M, Yang Y, Wang Y, Yuan Y, et al. Folate-mediated one-carbon metabolism: a targeting strategy in cancer therapy. Drug Discovery Today. 2021;26(3):817-25.
33. Simeone P, Tacconi S, Longo S, Lanuti P, Bravaccini S, Pirini F, et al. Expanding roles of de novo lipogenesis in breast cancer. International journal of environmental research and public health. 2021;18(7):3575.
34. Che L, Paliogiannis P, Cigliano A, Pilo MG, Chen X, Calvisi DF. Pathogenetic, prognostic, and therapeutic role of fatty acid synthase in human hepatocellular carcinoma. Frontiers in oncology. 2019;9:1412.
35. Piccinin E, Cariello M, De Santis S, Ducheix S, Sabbà C, Ntambi JM, et al. Role of oleic acid in the gut-liver axis: from diet to the regulation of its synthesis via stearoyl-CoA desaturase 1 (SCD1). Nutrients. 2019;11(10):2283.
36. Li B, Mi J, Yuan Q. Fatty acid metabolism-related enzymes in colorectal cancer metastasis: from biological function to molecular mechanism. Cell Death Discovery. 2024;10(1):350.
37. Schroeder B, Vander Steen T, Espinoza I, Venkatapoorna CMK, Hu Z, Silva FM, et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell death & disease. 2021;12(11):977.
38. Wang Z, Jiang Q, Dong C. Metabolic reprogramming in triple-negative breast cancer. Cancer biology & medicine. 2020;17(1):44-59.
39. Zheng Y, Jiang Z, Yuan L, Cheng X, He W, Chen X. Targeting fatty acid oxidation: A potential strategy for treating gastrointestinal tumors. International Journal of Cancer. 2025;157(1):7-17.
40. Ma Q, Kang R, Guan Y, Chang S, Li S. Crosstalk between stromal, immune, and ovarian cancer cells in lipid-rich tumor microenvironment exhibits proliferative features. Frontiers in Immunology. 2025;16:1614815.
41. Dewulf JP, Marie S, Nassogne M-C. Disorders of purine biosynthesis metabolism. Molecular genetics and metabolism. 2022;136(3):190-8.
42. Kitagawa Y, Kondo S, Fukuyo M, Wakae K, Dochi H, Mizokami H, et al. Phosphoribosyl pyrophosphate amidotransferase: Novel biomarker and therapeutic target for nasopharyngeal carcinoma. Cancer Science. 2024;115(11):3587-95.
43. Grotehans N. Nucleotide metabolism and the control of mitochondrial gene expression: Universität zu Köln; 2023.
44. Lu Z, Zhang C, Zhang J, Su W, Wang G, Wang Z. The Kynurenine Pathway and Indole Pathway in Tryptophan Metabolism Influence Tumor Progression. Cancer Medicine. 2025;14(6):e70703.
45. Kwiatkowska I, Hermanowicz JM, Przybyszewska-Podstawka A, Pawlak D. Not Only Immune Escape—The Confusing Role of the TRP Metabolic Pathway in Carcinogenesis. Cancers. 2021;13(11):2667.
46. Lin M, Zhou J, Xiao J, Li C, Mo Y, Liu Y, et al. Integrating multi-omics data of Triple-Negative Breast Cancer to explore the role of Kynurenine pathway and KYNU as a therapeutic target. Biochemical and Biophysical Research Communications. 2025;756:151569.
47. Anu R, Shiu K-K, Khan KH. The immunomodulatory role of IDO1-Kynurenine-NAD+ pathway in switching cold tumor microenvironment in PDAC. Frontiers in Oncology. 2023;13:1142838.
48. Jiang M, Fang H, Tian H. Metabolism of cancer cells and immune cells in the initiation, progression, and metastasis of cancer. Theranostics. 2025;15(1):155.
49. Hofer F, Di Sario G, Musiu C, Sartoris S, De Sanctis F, Ugel S. A complex metabolic network confers immunosuppressive functions to myeloid-derived suppressor cells (MDSCs) within the tumour microenvironment. Cells. 2021;10(10):2700.
50. Miranda-Galvis M, Teng Y. Targeting hypoxia-driven metabolic reprogramming to constrain tumor progression and metastasis. International journal of molecular sciences. 2020;21(15):5487.
51. Du W, Ren L, Hamblin MH, Fan Y. Endothelial cell glucose metabolism and angiogenesis. Biomedicines. 2021;9(2):147.
52. Farsani SSM, Verma V. Lactate mediated metabolic crosstalk between cancer and immune cells and its therapeutic implications. Frontiers in Oncology. 2023;13:1175532.
53. Sun Q, Wu J, Zhu G, Li T, Zhu X, Ni B, et al. Lactate-related metabolic reprogramming and immune regulation in colorectal cancer. Frontiers in Endocrinology. 2023;13:1089918.
54. Verma S, Budhu S, Serganova I, Dong L, Mangarin LM, Khan JF, et al. Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models. The Journal of Clinical Investigation. 2024;134(17).
55. Yin Z, Bai L, Li W, Zeng T, Tian H, Cui J. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. Journal of Experimental & Clinical Cancer Research. 2019;38(1):403.
56. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nature reviews cancer. 2020;20(9):516-31.
57. Aden D, Sureka N, Zaheer S, Chaurasia JK, Zaheer S. Metabolic reprogramming in cancer: implications for immunosuppressive microenvironment. Immunology. 2025;174(1):30-72.
58. Liu H, Pan M, Liu M, Zeng L, Li Y, Huang Z, et al. Lactate: a rising star in tumors and inflammation. Frontiers in Immunology. 2024;15:1496390.
59. Gu X-Y, Yang J-L, Lai R, Zhou Z-J, Tang D, Hu L, et al. Impact of lactate on immune cell function in the tumor microenvironment: Mechanisms and therapeutic perspectives. Frontiers in Immunology. 2025;16:1563303.
60. Iessi E, Vona R, Cittadini C, Matarrese P. Targeting the interplay between cancer metabolic reprogramming and cell death pathways as a viable therapeutic path. Biomedicines. 2021;9(12):1942.
61. Badran O, Cohen I, Bar-Sela G. Cancer-Associated Fibroblasts in Solid Tumors and Sarcomas: Heterogeneity, Function, and Therapeutic Implications. Cells. 2025;14(17):1398.
62. Zhou L, Zhang W, Hu X, Wang D, Tang D. Metabolic reprogramming of cancer-associated fibroblast in the tumor microenvironment: from basics to clinic. Clinical Medicine Insights: Oncology. 2024;18:11795549241287058.
63. Duatti A. Lactate-induced COL1A1/DDR1 axis promotes prostate cancer aggressiveness and enhances metastatic colonization. 2023.
64. Singh L, Nair L, Kumar D, Arora MK, Bajaj S, Gadewar M, et al. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Frontiers in Oncology. 2023;13:1034205.
65. Pereira-Nunes A, Afonso J, Granja S, Baltazar F. Lactate and lactate transporters as key players in the maintenance of the Warburg effect. Tumor microenvironment: the main driver of metabolic adaptation. 2020:51-74.
66. Dzhalilova DS, Makarova OV. HIF-dependent mechanisms of relationship between hypoxia tolerance and tumor development. Biochemistry (Moscow). 2021;86(10):1163-80.
67. Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal transduction and targeted therapy. 2023;8(1):70.
68. Bao MH-R, Wong CC-L. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. 2021;10(7):1715.
69. Ma S, Zhao Y, Lee WC, Ong L-T, Lee PL, Jiang Z, et al. Hypoxia induces HIF1α-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nature communications. 2022;13(1):4118.
70. Sieminska I, Baran J. Myeloid-derived suppressor cells in colorectal cancer. Frontiers in immunology. 2020;11:1526.