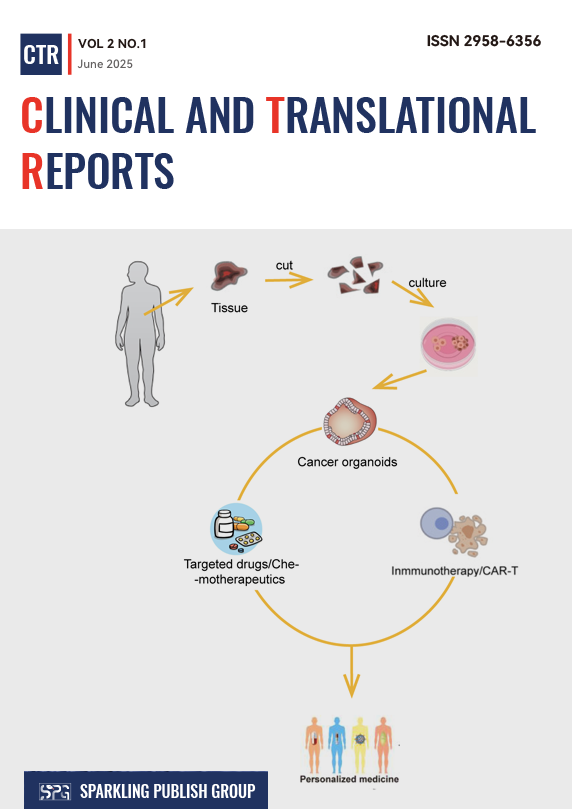
Organoids, three-dimensional (3D) in vitro models derived from patient tissues or stem cells, have developed into transformative tools in oncology by recapitulating the structural, genetic, and functional heterogeneity of native tumors. move from fundamental research to clinical application. This review outlines key applications of organoids in cancer research, including tumor modeling, high-throughput drug screening, immunotherapy evaluation, and dissection of metastatic mechanisms. Case studies of various cancers highlight their ability to preserve patient-specific genomic alterations and predict treatment outcomes. Although challenges remain in many areas, multidisciplinary advances in bioengineering and multi-omics integration are expected to overcome these limitations, demonstrating the unprecedented potential of this technology.
1. Ho BX, Pek NMQ, Soh B-S. Disease modeling using 3D organoids derived from human induced pluripotent stem cells. International journal of molecular sciences. 2018;19(4):936.
2. Tang X-Y, Wu S, Wang D, Chu C, Hong Y, Tao M, et al. Human organoids in basic research and clinical applications. Signal transduction and targeted therapy. 2022;7(1):168.
3. Sajjad H, Imtiaz S, Noor T, Siddiqui YH, Sajjad A, Zia M. Cancer models in preclinical research: A chronicle review of advancement in effective cancer research. Animal models and experimental medicine. 2021;4(2):87-103.
4. Yee C, Dickson K-A, Muntasir MN, Ma Y, Marsh DJ. Three-dimensional modelling of ovarian cancer: from cell lines to organoids for discovery and personalized medicine. Frontiers in Bioengineering and Biotechnology. 2022;10:836984.
5. Monro S, Colon KL, Yin H, Roque III J, Konda P, Gujar S, et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chemical reviews. 2018;119(2):797-828.
6. Liu B, Zhou H, Tan L, Siu KTH, Guan X-Y. Exploring treatment options in cancer: tumor treatment strategies. Signal transduction and targeted therapy. 2024;9(1):175.
7. Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Archives of medical science. 2018;14(4):910-9.
8. Wang H, Brown PC, Chow EC, Ewart L, Ferguson SS, Fitzpatrick S, et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clinical and translational science. 2021;14(5):1659-80.
9. Chunduri V, Maddi S. Role of in vitro two-dimensional (2D) and three-dimensional (3D) cell culture systems for ADME-Tox screening in drug discovery and development: a comprehensive review. ADMET and DMPK. 2023;11(1):1-32.
10. Artero Castro A, Rodríguez Jimenez FJ, Jendelova P, Erceg S. Deciphering retinal diseases through the generation of three dimensional stem cell-derived organoids: Concise Review. Stem Cells. 2019;37(12):1496-504.
11. Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L, et al. Organoids. Nature Reviews Methods Primers. 2022;2(1):94.
12. Hofer M, Lutolf MP. Engineering organoids. Nature Reviews Materials. 2021;6(5):402-20.
13. LeSavage BL, Suhar RA, Broguiere N, Lutolf MP, Heilshorn SC. Next-generation cancer organoids. Nature materials. 2022;21(2):143-59.
14. Es HA, Montazeri L, Aref AR, Vosough M, Baharvand H. Personalized cancer medicine: an organoid approach. Trends in biotechnology. 2018;36(4):358-71.
15. Gao M, Lin M, Rao M, Thompson H, Hirai K, Choi M, et al. Development of patient-derived gastric cancer organoids from endoscopic biopsies and surgical tissues. Annals of surgical oncology. 2018;25:2767-75.
16. Mazzucchelli S, Piccotti F, Allevi R, Truffi M, Sorrentino L, Russo L, et al. Establishment and morphological characterization of patient-derived organoids from breast cancer. Biological procedures online. 2019;21:1-13.
17. Jacob F, Ming G-l, Song H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nature Protocols. 2020;15(12):4000-33.
18. Xu R, Zhou X, Wang S, Trinkle C. Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacology & therapeutics. 2021;218:107668.
19. Devarasetty M, Forsythe SD, Shelkey E, Soker S. In vitro modeling of the tumor microenvironment in tumor organoids. Tissue Engineering and Regenerative Medicine. 2020;17:759-71.
20. Jeong S-R, Kang M. Exploring Tumor–Immune Interactions in Co-Culture Models of T Cells and Tumor Organoids Derived from Patients. International Journal of Molecular Sciences. 2023;24(19):14609.
21. Qu J, Kalyani FS, Liu L, Cheng T, Chen L. Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Communications. 2021;41(12):1331-53.
22. Luo Z, Zhou X, Mandal K, He N, Wennerberg W, Qu M, et al. Reconstructing the tumor architecture into organoids. Advanced drug delivery reviews. 2021;176:113839.
23. Polak R, Zhang ET, Kuo CJ. Cancer organoids 2.0: modelling the complexity of the tumour immune microenvironment. Nature Reviews Cancer. 2024;24(8):523-39.
24. Xiu Z, Yang Q, Xie F, Han F, He W, Liao W. Revolutionizing digestive system tumor organoids research: Exploring the potential of tumor organoids. Journal of Tissue Engineering. 2024;15:20417314241255470.
25. Vella N, Fenech AG, Petroni Magri V. 3D cell culture models in research: applications to lung cancer pharmacology. Frontiers in Pharmacology. 2024;15:1438067.
26. Ma X, Wang Q, Li G, Li H, Xu S, Pang D. Cancer organoids: A platform in basic and translational research. Genes & diseases. 2024;11(2):614-32.
27. Huang J, Wang X, Ge S, Lu X, Sun C. Organoids as Sophisticated Tools for Renal Cancer Research: Extensive Applications and Promising Prospects. Cellular and Molecular Bioengineering. 2024:1-22.
28. Ramakrishna G, Babu PE, Singh R, Trehanpati N. Application of CRISPR-Cas9 based gene editing to study the pathogenesis of colon and liver cancer using organoids. Hepatology international. 2021:1-9.
29. Poghosyan S, Frenkel N, Lentzas A, Laoukili J, Rinkes IB, Kranenburg O, et al. Loss of neuropilin-2 in murine mesenchymal-like colon cancer organoids causes mesenchymal-to-Epithelial transition and an acquired dependency on insulin-receptor signaling and autophagy. Cancers. 2022;14(3):671.
30. Liu G, Xiao X, Xia Y, Huang W, Chen W, Xu J, et al. Organoids from mucinous appendiceal adenocarcinomas as high-fidelity models for individual therapy. Frontiers in Medicine. 2022;9:829033.
31. Zhou Z, Cong L, Cong X. Patient-derived organoids in precision medicine: drug screening, organoid-on-a-chip and living organoid biobank. Frontiers in Oncology. 2021;11:762184.
32. Luo Z, Wang B, Luo F, Guo Y, Jiang N, Wei J, et al. Establishment of a large-scale patient-derived high-risk colorectal adenoma organoid biobank for high-throughput and high-content drug screening. BMC medicine. 2023;21(1):336.
33. Yuan J, Li X, Yu S. Cancer organoid co-culture model system: Novel approach to guide precision medicine. Frontiers in Immunology. 2023;13:1061388.
34. Dalir Abdolahinia E, Han X. The three-dimensional in vitro cell culture models in the study of oral cancer immune microenvironment. Cancers. 2023;15(17):4266.
35. Chakraborty S, DePalma TJ, Skardal A. Increasing accuracy of in vitro cancer models: engineering stromal complexity into tumor organoid platforms. Advanced NanoBiomed Research. 2021;1(12):2100061.
36. Cadavid Cardenas JL. An Engineered Paper-Based 3D Co-Culture Model of Pancreatic Cancer as a Platform for Systems Tissue Engineering 2023.
37. Wang G, Zhang M, Cheng M, Wang X, Li K, Chen J, et al. Tumor microenvironment in head and neck squamous cell carcinoma: Functions and regulatory mechanisms. Cancer Letters. 2021;507:55-69.
38. Simiczyjew A, Dratkiewicz E, Mazurkiewicz J, Ziętek M, Matkowski R, Nowak D. The influence of tumor microenvironment on immune escape of melanoma. International Journal of Molecular Sciences. 2020;21(21):8359.
39. Tian H, Ren J, Mou R, Jia Y. Application of organoids in precision immunotherapy of lung cancer. Oncology Letters. 2023;26(5):1-11.
40. Garnett M. Werner helicase is a synthetic-lethal vulnerability in Mismatch Repair–Deficient Colorectal Cancer Refractory to Targeted Therapies, Chemotherapy and Immunotherapy. 2024.
41. Zhou Z, Pang Y, Ji J, He J, Liu T, Ouyang L, et al. Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies. Nature Reviews Immunology. 2024;24(1):18-32.
42. Zeng J, Li X, Sander M, Zhang H, Yan G, Lin Y. Oncolytic viro-immunotherapy: an emerging option in the treatment of gliomas. Frontiers in immunology. 2021;12:721830.
43. Magré L, Verstegen MM, Buschow S, van der Laan LJ, Peppelenbosch M, Desai J. Emerging organoid-immune co-culture models for cancer research: from oncoimmunology to personalized immunotherapies. Journal for ImmunoTherapy of Cancer. 2023;11(5).
44. Kong JCH, Guerra GR, Millen RM, Roth S, Xu H, Neeson PJ, et al. Tumor-infiltrating lymphocyte function predicts response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. JCO Precision oncology. 2018;2:1-15.
45. Mannan A, Kakkar C, Dhiman S, Singh TG. Advancing the frontiers of adaptive cell therapy: A transformative mechanistic journey from preclinical to clinical settings. International Immunopharmacology. 2023;125:111095.
46. Dagar G, Gupta A, Masoodi T, Nisar S, Merhi M, Hashem S, et al. Harnessing the potential of CAR-T cell therapy: progress, challenges, and future directions in hematological and solid tumor treatments. Journal of translational medicine. 2023;21(1):449.
47. Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Frontiers in immunology. 2019;10:2250.
48. Yu L, Li Z, Mei H, Li W, Chen D, Liu L, et al. Patient‐derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR‐T cells in vitro. Clinical & translational immunology. 2021;10(2):e1248.
49. Riggi N, Aguet M, Stamenkovic I. Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annual Review of Pathology: Mechanisms of Disease. 2018;13(1):117-40.
50. Isci D. Multilevel investigation of the atypical chemokine receptor 3 (ACKR3) expression and function in glioblastoma: Universite de Liege (Belgium); 2024.
51. Gil JS. Design and Characterization of A CXCR4-Retargeted and Strailarmed Oncolytic HSV-1 to Attack Glioblastoma Stem-Like Cells (GSCs): Universite de Liege (Belgium); 2022.
52. Roe J-S, Hwang C-I, Somerville TD, Milazzo JP, Lee EJ, Da Silva B, et al. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell. 2017;170(5):875-88. e20.
53. Compagni A, Christofori G. Recent advances in research on multistage tumorigenesis. British journal of cancer. 2000;83(1):1-5.
54. Tavassoli M, Pezzella F. Oncogenesis and tumour suppression. Oxford Textbook of Cancer Biology. 2019:136.
55. Devi PU. Basics of carcinogenesis. Health Adm. 2004;17(1):16-24.
56. Zhao Y, Shen M, Wu L, Yang H, Yao Y, Yang Q, et al. Stromal cells in the tumor microenvironment: accomplices of tumor progression? Cell Death & Disease. 2023;14(9):587.
57. Parsa N. Environmental factors inducing human cancers. Iranian journal of public health. 2012;41(11):1.
58. Zhang J, Wang L, Song Q, Xiao M, Gao J, Cao X, et al. Organoids in recapitulating tumorigenesis driven by risk factors: Current trends and future perspectives. International Journal of Biological Sciences. 2022;18(7):2729.
59. Xu H, Jiao D, Liu A, Wu K. Tumor organoids: applications in cancer modeling and potentials in precision medicine. Journal of hematology & oncology. 2022;15(1):58.
60. Jefremow A, Neurath MF, Waldner MJ. CRISPR/Cas9 in gastrointestinal malignancies. Frontiers in Cell and Developmental Biology. 2021;9:727217.
61. Takeda H, Kataoka S, Nakayama M, Ali MA, Oshima H, Yamamoto D, et al. CRISPR-Cas9–mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proceedings of the National Academy of Sciences. 2019;116(31):15635-44.
62. Yan S, Wang S, Wang X, Dai W, Chu J, Cheng M, et al. Emerging role of non-coding RNAs in glucose metabolic reprogramming and chemoresistance in colorectal cancer. Frontiers in Oncology. 2022;12:954329.
63. Campaner E, Zannini A, Santorsola M, Bonazza D, Bottin C, Cancila V, et al. Breast cancer organoids model patient-specific response to drug treatment. Cancers. 2020;12(12):3869.
64. Huang S, Mei Z, Wan A, Zhao M, Qi X. Application and prospect of organoid technology in breast cancer. Frontiers in Immunology. 2024;15:1413858.
65. Plava J, Cehakova M, Kuniakova M, Trnkova L, Cihova M, Bohac M, et al. The third dimension of tumor microenvironment—The importance of tumor stroma in 3D cancer models. Experimental Biology and Medicine. 2023;248(15):1347-58.
66. Bayat M, Nahand JS. Let’s make it personal: CRISPR tools in manipulating cell death pathways for cancer treatment. Cell Biology and Toxicology. 2024;40(1):61.
67. Yang Q, Li M, Yang X, Xiao Z, Tong X, Tuerdi A, et al. Flourishing tumor organoids: History, emerging technology, and application. Bioengineering & Translational Medicine. 2023;8(5):e10559.
68. Zeng G, Yu Y, Wang M, Liu J, He G, Yu S, et al. Advancing cancer research through organoid technology. Journal of Translational Medicine. 2024;22(1):1007.
69. Nie D, Guo T, Yue M, Li W, Zong X, Zhu Y, et al. Research progress on nanoparticles-based CRISPR/Cas9 system for targeted therapy of tumors. Biomolecules. 2022;12(9):1239.
70. Radhakrishnan J, Varadaraj S, Dash SK, Sharma A, Verma RS. Organotypic cancer tissue models for drug screening: 3D constructs, bioprinting and microfluidic chips. Drug Discovery Today. 2020;25(5):879-90.
71. Shirure VS, Hughes CC, George SC. Engineering vascularized organoid-on-a-chip models. Annual Review of Biomedical Engineering. 2021;23(1):141-67.
72. Jung M, Ghamrawi S, Du EY, Gooding JJ, Kavallaris M. Advances in 3D bioprinting for cancer biology and precision medicine: From matrix design to application. Advanced Healthcare Materials. 2022;11(24):2200690.
73. Gu Q, Tomaskovic‐Crook E, Wallace GG, Crook JM. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Advanced healthcare materials. 2017;6(17):1700175.
74. Orlow I. Molecular Mechanism of Carcinogenesis: Insight into Tumor Initiation and Progression. Journal of Carcinogenesis. 2024;23(1).
75. Cekanova M, Rathore K. Animal models and therapeutic molecular targets of cancer: utility and limitations. Drug design, development and therapy. 2014:1911-22.
76. Cigliano A, Liao W, Deiana GA, Rizzo D, Chen X, Calvisi DF. Preclinical Models of Hepatocellular Carcinoma: Current Utility, Limitations, and Challenges. Biomedicines. 2024;12(7):1624.
77. Ciucci A, Buttarelli M, Fagotti A, Scambia G, Gallo D. Preclinical models of epithelial ovarian cancer: practical considerations and challenges for a meaningful application. Cellular and Molecular Life Sciences. 2022;79(7):364.
78. Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito C, Gutekunst M, et al. Three‐dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnology journal. 2014;9(9):1115-28.
79. Fong EL, Harrington DA, Farach-Carson MC, Yu H. Heralding a new paradigm in 3D tumor modeling. Biomaterials. 2016;108:197-213.
80. Tong CW, Wu M, Cho WC, To KK. Recent advances in the treatment of breast cancer. Frontiers in oncology. 2018;8:227.
81. Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, et al. Patient‐derived organoids can guide personalized‐therapies for patients with advanced breast cancer. Advanced Science. 2021;8(22):2101176.
82. Würth R, Donato E, Michel LL, Saini M, Becker L, Cheytan T, et al. Circulating tumor cell plasticity determines breast cancer therapy resistance via neuregulin 1–HER3 signaling. Nature cancer. 2025:1-19.
83. Li H, Wang J, Yi Z, Li C, Wang H, Zhang J, et al. CDK12 inhibition enhances sensitivity of HER2+ breast cancers to HER2-tyrosine kinase inhibitor via suppressing PI3K/AKT. European Journal of Cancer. 2021;145:92-108.
84. Salemme V, Centonze G, Avalle L, Natalini D, Piccolantonio A, Arina P, et al. The role of tumor microenvironment in drug resistance: emerging technologies to unravel breast cancer heterogeneity. Frontiers in Oncology. 2023;13:1170264.
85. Svanström A, Rosendahl J, Salerno S, Leiva MC, Gregersson P, Berglin M, et al. Optimized alginate-based 3D printed scaffolds as a model of patient derived breast cancer microenvironments in drug discovery. Biomedical Materials. 2021;16(4):045046.
86. Van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933-45.
87. Janney A, Powrie F, Mann EH. Host–microbiota maladaptation in colorectal cancer. Nature. 2020;585(7826):509-17.
88. Gjeta B. Unveiling cellular dynamics: exploring human disease progression and therapeutic potential through organoid models and single-cell technologies: University_of_Basel; 2023.
89. Tran F, Klein C, Arlt A, Imm S, Knappe E, Simmons A, et al. Stem cells and organoid technology in precision medicine in inflammation: are we there yet? Frontiers in immunology. 2020;11:573562.
90. Zheng H, Liu J, Cheng Q, Zhang Q, Zhang Y, Jiang L, et al. Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance. Nature Cancer. 2024:1-18.
91. Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J, et al. 3D model for CAR‐mediated cytotoxicity using patient‐derived colorectal cancer organoids. The EMBO journal. 2019;38(12):e100928.
92. Testa U, Castelli G, Pelosi E. Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers. 2018;10(8):248.
93. Liu Y, Zhou Y, Chen P. Lung cancer organoids: models for preclinical research and precision medicine. Frontiers in Oncology. 2023;13:1293441.
94. Lee EJ, Oh SY, Lee YW, Kim JY, Kim M-J, Kim TH, et al. Discovery of a novel potent EGFR inhibitor against EGFR activating mutations and on-target resistance in NSCLC. Clinical Cancer Research. 2024;30(8):1582-94.
95. Kim M, Mun H, Sung CO, Cho EJ, Jeon H-J, Chun S-M, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nature communications. 2019;10(1):3991.
96. Takahashi N, Hoshi H, Higa A, Hiyama G, Tamura H, Ogawa M, et al. An in vitro system for evaluating molecular targeted drugs using lung patient-derived tumor organoids. Cells. 2019;8(5):481.
97. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World journal of gastroenterology. 2018;24(43):4846.
98. Raghavan S, Winter PS, Navia AW, Williams HL, DenAdel A, Lowder KE, et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell. 2021;184(25):6119-37. e26.
99. Navarro Serer B. INTERROGATING THE ROLE OF MOLECULAR ALTERATIONS AND THE TUMOR MICROENVIRONMENT ON PANCREATIC DUCTAL ADENOCARCINOMA INVASION IN A THREE-DIMENSIONAL HUMAN ORGANOID MODEL: Johns Hopkins University; 2022.
100. Holokai L, Chakrabarti J, Lundy J, Croagh D, Adhikary P, Richards SS, et al. Murine-and human-derived autologous organoid/immune cell co-cultures as pre-clinical models of pancreatic ductal adenocarcinoma. Cancers. 2020;12(12):3816.
101. Poulia KA, Sarantis P, Antoniadou D, Koustas E, Papadimitropoulou A, Papavassiliou AG, et al. Pancreatic cancer and cachexia—metabolic mechanisms and novel insights. Nutrients. 2020;12(6):1543.
102. Vaes RD, van Dijk DP, Welbers TT, Blok MJ, Aberle MR, Heij L, et al. Generation and initial characterization of novel tumour organoid models to study human pancreatic cancer‐induced cachexia. Journal of cachexia, sarcopenia and muscle. 2020;11(6):1509-24.
103. Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nature medicine. 2017;23(12):1424-35.
104. Castven D. Patient-derived cancer cells to dissect the molecular basis of treatment response in primary liver cancer: a mechanistic and functional approach: Johannes Gutenberg-Universität Mainz; 2019.
105. Xu F, Tong M, Tong CS, Chan BK, Chu HY, Wong TL, et al. A combinatorial CRISPR–Cas9 screen identifies ifenprodil as an adjunct to sorafenib for liver cancer treatment. Cancer Research. 2021;81(24):6219-32.
106. Simon SM. Fighting rare cancers: lessons from fibrolamellar hepatocellular carcinoma. Nature Reviews Cancer. 2023;23(5):335-46.
107. Song J, Lu M, He Z, Zhang W. Models of fibrolamellar carcinomas, tools for evaluation of a new era of treatments. Frontiers in Immunology. 2024;15:1459942.
108. Rüland L, Andreatta F, Massalini S, Chuva de Sousa Lopes S, Clevers H, Hendriks D, et al. Organoid models of fibrolamellar carcinoma mutations reveal hepatocyte transdifferentiation through cooperative BAP1 and PRKAR2A loss. Nature Communications. 2023;14(1):2377.
109. Gulati R, Johnston M, Rivas M, Cast A, Kumbaji M, Hanlon MA, et al. β‐catenin cancer–enhancing genomic regions axis is involved in the development of fibrolamellar hepatocellular carcinoma. Hepatology Communications. 2022;6(10):2950-63.
110. Beltran L, Berney DM. The urinary tract and male reproductive system. Muir's Textbook of Pathology: CRC Press; 2020. p. 447-62.
111. Karkampouna S, La Manna F, Benjak A, Kiener M, De Menna M, Zoni E, et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nature communications. 2021;12(1):1117.
112. Beshiri ML, Tice CM, Tran C, Nguyen HM, Sowalsky AG, Agarwal S, et al. A PDX/organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening. Clinical Cancer Research. 2018;24(17):4332-45.
113. Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chinese clinical oncology. 2020;9(4):47-.
114. Kopper O, De Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, et al. An organoid platform for ovarian cancer captures intra-and interpatient heterogeneity. Nature medicine. 2019;25(5):838-49.
115. Tao M, Wu X. The role of patient-derived ovarian cancer organoids in the study of PARP inhibitors sensitivity and resistance: from genomic analysis to functional testing. Journal of Experimental & Clinical Cancer Research. 2021;40(1):338.
116. Nero C, Vizzielli G, Lorusso D, Cesari E, Daniele G, Loverro M, et al. Patient-derived organoids and high grade serous ovarian cancer: from disease modeling to personalized medicine. Journal of Experimental & Clinical Cancer Research. 2021;40:1-14.
117. Zhang M, Yin R, Li K. Advances and challenges in the origin and evolution of ovarian cancer organoids. Frontiers in Oncology. 2024;14:1429141.
118. Brodeur M. Predictive carboplatin treatment response models for epithelial ovarian cancer: comparison of 2D, 3D and in-vivo models. 2021.
119. Matsuoka T, Yashiro M. Precision medicine for gastrointestinal cancer: Recent progress and future perspective. World journal of gastrointestinal oncology. 2020;12(1):1.
120. Yan HH, Siu HC, Law S, Ho SL, Yue SS, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell stem cell. 2018;23(6):882-97. e11.
121. Chen G, Han R, Wang L, Ma W, Zhang W, Lu Z, et al. Establishment of patient-derived organoids and a characterization based drug discovery platform for treatment of gastric cancer. Cancer Cell International. 2024;24(1):288.
122. Jeon J, Cheong J-H. Clinical implementation of precision medicine in gastric cancer. Journal of gastric cancer. 2019;19(3):235-53.
123. Xu J, Yu B, Wang F, Yang J. Xenograft and organoid models in developing precision medicine for gastric cancer. International Journal of Oncology. 2024;64(4):1-16.
124. Schaff LR, Mellinghoff IK. Glioblastoma and other primary brain malignancies in adults: a review. Jama. 2023;329(7):574-87.
125. Jacob F. Modeling Human Brain Development and Disease Using Primary Glioblastoma and Human Stem Cell-derived Organoids: The Johns Hopkins University; 2020.
126. Bosio AG. IDENTIFICATION OF NOVEL BIGUANIDE BASEDCOMPOUNDS AS ANTI TUMOR AGENTS FOR GLIOBLASTOMA: PHARMACOLOGICAL ASSESSMENT IN 3D CULTURE MODELS. 2022.
127. Luksik AS, Yazigi E, Shah P, Jackson CM. CAR T cell therapy in glioblastoma: overcoming challenges related to antigen expression. Cancers. 2023;15(5):1414.
128. Filippini DM, Carosi F, Querzoli G, Fermi M, Ricciotti I, Molteni G, et al. Rare Head and Neck Cancers and Pathological Diagnosis Challenges: A Comprehensive Literature Review. Diagnostics. 2024;14(21):2365.
129. Millen R, De Kort WW, Koomen M, van Son GJ, Gobits R, de Vries BP, et al. Patient-derived head and neck cancer organoids allow treatment stratification and serve as a tool for biomarker validation and identification. Med. 2023;4(5):290-310. e12.
130. Driehuis E, Kretzschmar K, Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nature protocols. 2020;15(10):3380-409.
131. Sobczuk P, Brodziak A, Khan MI, Chhabra S, Fiedorowicz M, Wełniak-Kamińska M, et al. Choosing the right animal model for renal cancer research. Translational oncology. 2020;13(3):100745.
132. Li Z, Xu H, Yu L, Wang J, Meng Q, Mei H, et al. Patient‐derived renal cell carcinoma organoids for personalized cancer therapy. Clinical and Translational Medicine. 2022;12(7):e970.
133. Xue Y, Wang B, Tao Y, Xia J, Yuan K, Zheng J, et al. Patient-derived organoids potentiate precision medicine in advanced clear cell renal cell carcinoma. Precision Clinical Medicine. 2022;5(4):pbac028.
134. Rupert C, Dell’Aversana C, Mosca L, Montanaro V, Arcaniolo D, De Sio M, et al. Therapeutic targeting of P2X4 receptor and mitochondrial metabolism in clear cell renal carcinoma models. Journal of Experimental & Clinical Cancer Research. 2023;42(1):134.
135. Feng Q-S, Shan X-F, Yau V, Cai Z-G, Xie S. Facilitation of Tumor Stroma-Targeted Therapy: Model Difficulty and Co-Culture Organoid Method. Pharmaceuticals. 2025;18(1):62.
136. Chen H, Cheng Y, Wang X, Wang J, Shi X, Li X, et al. 3D printed in vitro tumor tissue model of colorectal cancer. Theranostics. 2020;10(26):12127.
137. Bhattacharya A, Alam K, Roy NS, Kaur K, Kaity S, Ravichandiran V, et al. Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer. Journal of Experimental & Clinical Cancer Research. 2023;42(1):343.
138. Gu Z, Wu Q, Shang B, Zhang K, Zhang W. Organoid co‐culture models of the tumor microenvironment promote precision medicine. Cancer Innovation. 2024;3(1):e101.
139. Lê H, Seitlinger J, Lindner V, Olland A, Falcoz P-E, Benkirane-Jessel N, et al. Patient-Derived Lung Tumoroids—An Emerging Technology in Drug Development and Precision Medicine. Biomedicines. 2022;10(7):1677.
140. Wang J, Chen C, Wang L, Xie M, Ge X, Wu S, et al. Patient-derived tumor organoids: new progress and opportunities to facilitate precision cancer immunotherapy. Frontiers in Oncology. 2022;12:872531.
141. Gu A, Li J, Qiu S, Hao S, Yue Z-Y, Zhai S, et al. Pancreatic cancer environment: from patient-derived models to single-cell omics. Molecular Omics. 2024;20(4):220-33.